Abstract
Background: Crosstalk between the bone marrow microenvironment and leukemia cells plays a pivotal role in pathogenesis. Mesenchymal stem cells (MSCs) acquire defects during natural ageing, with reduced regenerative potential and functional impairment. Since chronic myelomonocytic leukemia (CMML) mainly affects older adults, defective MSCs could play a critical and targetable role in disease initiation and progression. Identifying molecular abnormalities associated with CMML MSCs would reveal novel therapeutic targets for patient benefit.
Methods: Phenotypes of age-matched healthy and CMML MSCs were compared by a range of functional approaches, including senescence, multilineage differentiation and hematopoietic stem cell maintenance assays. To identify altered epigenetic and transcriptional networks we performed RNA-Seq (n=18 CMML/10 healthy) and ATAC-Seq (n=12 CMML/5 healthy) on cultured MSCs (CD44+73+90+105+146+), integrating datasets to explore gene regulatory networks operational within CMML MSCs. To investigate direct crosstalk between MSCs and malignant cells we performed RNA-Seq on healthy MSCs following 7 days co-culture with CMML blood mononuclear cells (MNCs). Functional validation was performed through inhibitor studies using apoptosis, senescence, osteogenesis, and stem cell maintenance as readouts.
Results: CMML MSCs displayed consistent and profound intrinsic growth defects, with higher senescence and apoptosis, and lower proliferation and clonogenic capacity. CMML MSCs also displayed a marked differentiation bias, with increased osteogenic lineage potential but reduced adipogenicity and chondrogenicity. Importantly, CMML MSCs demonstrated reduced stem cell maintenance capability in co-culture experiments, confirming a functional consequence on hematopoiesis.
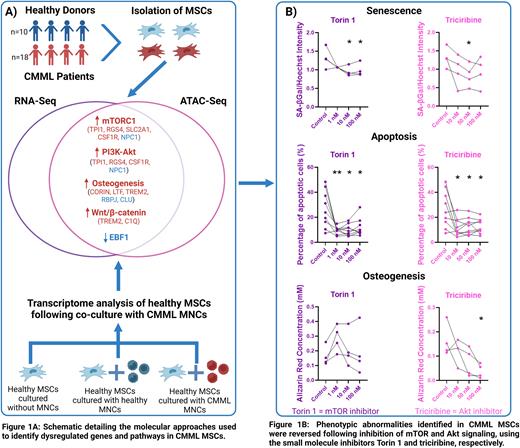
Transcriptomic analysis showed activation of novel pathways including mTOR, PI3K-Akt signaling, and oxidative phosphorylation in CMML MSCs, in addition to Wnt pathway upregulation as shown previously (Figure 1A). Consistent with functional readouts, CMML MSCs were transcriptionally primed towards the osteo-lineage, with high expression of osteogenic promoting factors (CORIN, LTF, TREM2, SMAD5, ALX5) and downregulation of osteogenic inhibitors (RBPJ, CLU).
In line with transcriptomic analysis, ATAC-Seq of CMML MSCs highlighted aberrantly accessible chromatin regions linked to genes involved in mTOR, PI3K-Akt and Wnt signaling as well as promotion of osteogenesis (Figure 1A). Integration of datasets identified downregulated expression and activity of the transcription factor EBF1, where reduced expression is associated with self-renewal defects, decreased adipogenesis, cellular senescence and defective stem cell maintenance ability, compatible with our observations in CMML MSCs. Similarly, we identified downregulated activity of the transcription factor RBPJ, potentially mediating the preferential enhancement of osteogenesis observed in CMML MSCs.
Following co-culture with CMML MNCs, healthy MSCs showed activation of mTOR, PI3K-Akt and osteogenesis, verifying a direct influence of leukemic cells on MSCs. Remarkably, integration of all datasets identified consistently upregulated genes (RGS4, TPI1) which positively regulate mTOR and PI3K-Akt signaling. Inhibiting these aberrantly active pathways in CMML MSCs using small molecule modulators significantly reversed the phenotypic abnormalities with a reduction in apoptosis, senescence and osteogenesis and restoration of their ability to support and maintain stem cells (Figure 1B).
Discussion: Our integrated analysis identified altered gene regulatory networks that are linked to abnormal pathways active in CMML MSCs, which can be targeted to restore their normal function. These results highlight novel pathogenic roles for non-clonal MSCs in the CMML microenvironment, and potential adjunctive strategies for targeting microenvironmental alongside cell intrinsic vulnerabilities.
Disclosures
Somervaille:Abbvie: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Imago Biosciences: Research Funding; Oryzon Genomics: Consultancy; CellCentric Ltd: Research Funding. Wiseman:Blueprint Oncology: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria; Astex Pharmaceuticals: Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.